
Many medicines are biologics. It means, that they are obtained from living sources, for example, Cells. Biologics are complex mixtures, molecules of active components of which (usually proteins), hundreds of times larger than the molecules of most traditional drugs. Usually, these drugs are only effective when given intravenously..
Biological products include blood and its components, vaccines, as well as recombinant proteins and monoclonal antibodies obtained using biotechnological methods. Most biologics are subject to the jurisdiction of the FDA, regulated by the Public Health Services Act and requires a biologic license prior to being marketed (biologic license application, BLA).
In the late 90s, biotechnology was associated mainly with recombinant biologics and antibodies, however, biotech companies are now increasingly relying on discoveries in genetics and other areas of biology to develop so-called small molecules. Such drugs with a simple chemical structure are often produced in tablet form. (although there are also injection and infusion forms). Besides, upon expiration of the license, their chemical structure is easily reproduced by generic companies.
The FDA regulates the manufacture of small molecule drugs under the FDA, medicines and cosmetics. To release new drugs on the market, you must obtain approval for their use.. (Production and use of certain biological products, including insulin and growth hormone, also governed by this law.)
Although the production of “conventional” drugs and biologics is regulated by different laws and regulations, all drugs and most therapeutic biologicals fall under the authority of the Center for the Study and Testing of Biologicals (Center for Biologics Evaluation and Research, CBER), part of the Food and Drug Administration (FDA).
Drug testing
On average, the development of a new therapy requires 10-15 years and more 800 million US dollars (taking into account the cost of unsuccessful attempts). The process of testing the safety and efficacy of a new drug is extremely complex and consists of many stages.. It usually starts with laboratory and animal testing., followed by human clinical trials, regulatory testing and, in case of product approval, post-marketing research and observation.
Animal testing
Once a potential drug has been identified, the next step is usually animal testing., which are carried out using at least two types, because. the drug can have different effects on representatives of different species. Most of these tests are essentially toxicological studies or ADME - Absorption studies., Distribution, Metabolism and Excretion (suction, distribution, metabolism, excretion). When they are carried out, the absorption of the drug is studied., breakdown mechanisms in the body, toxicity and activity of decomposition products (metabolites), as well as the rate of elimination of the drug itself and its metabolites from the body.
Scientists are also using animal models of various diseases to test the effects of drugs.. The results of such trials help with further drug improvement and clinical trials.. Despite, that testing the effectiveness of drugs in animals is an important step in the development process, its results are a recommendation for FDA approval for clinical use only when products are reviewed, designed to ensure biological safety. The safety of such products can be tested in humans., however, testing their effectiveness is not ethically acceptable, because. it involves exposing volunteers to a chemical warfare agent or anthrax agent to determine the effectiveness of a vaccine or drug.
Over time, scientists hope to supplement or partially replace animal testing with other techniques., for example, computer modeling of biological mechanisms. However, a specific set of animal experiments is necessary to ensure maximum drug safety prior to clinical trials.. BIO members report to Ethical principles BIO on the use and care of animals in biotechnological research.
Clinical trials
Upon successful completion of animal safety tests of the medicinal product, pre-registering a new drug application with the FDA, in clinical trial (investigational new drug, IND), clinical trials can begin. From the day of registration of the application to the start of testing, at least 30 days.
Each new drug goes through several stages of clinical trials. Early trials (phase 1) are to test the safety of the drug on small groups of healthy volunteers. The goal of larger interim tests (phase 2) is to obtain preliminary data on the effectiveness. The final stage of pre-marketing testing (phase 3) carried out to obtain convincing data on the effectiveness of the drug in relation to the target patient population, for which it is intended to cure.
The clinical trial design or protocol can vary significantly depending on the nature of the drug., patient population and the effectiveness of existing treatments.
Clinical trials of some drugs, intended for the treatment of rare and serious diseases, allowed to conduct on small groups of patients. In the same time, medicines for milder conditions and / or when alternative treatments exist, needs to be tested on thousands of patients to obtain official approval.
In many tests (especially in the absence of effective treatments) one group of patients (experimental group) receives the test drug, and the other (control group) - placebo, not different from the drug in any appearance, nor by the method of introduction. The division of patients into groups is carried out randomly..
Test method, in which the executing doctors know, which patient is receiving the active drug, and who is a placebo, called one-way blind. Respectively, double-blind method is called an approach, in which the distribution of patients into groups is not known to the investigators, nor test participants. Double-blind is considered the gold standard for clinical trials, especially evaluating the effectiveness of drugs.
Other key terms, relating to clinical trials:
Testers – doctors or other health professionals, conducting tests.
Medical Ethics Commissions – teams of specialists, monitoring the ethics and safety of testing at the local level (at the clinic level, university, etc.).
End points – measures to evaluate the results of a clinical trial (for example, reduction in tumor size, absence of a virus in the body, survival rate).
Indication – specific condition, for the treatment of which the drug is intended; the reading can be wide (for example, diabetes) or narrow (for example, insulin-dependent diabetes 1 Sort of).
Adequate work planning is essential to obtain interpretable clinical trial results, that is, recruiting a sufficient number of healthy volunteers or patients and using well-chosen endpoints.
The data obtained at the end of a well-designed test are recorded and analyzed.. The significance of the results is assessed using the statistical criterion p (significance level, or credibility). This indicator is a calculated value of the probability that, that the test results are the result of a coincidence. For example, at p = 0.01 there is 1% probability of randomness of results. Clinical trials are generally considered successful, if for its endpoints p<0,05, which corresponds to less 5% probability of randomness of results.
Phase 1
The first trial of a medicinal product or biological product - phase 1 clinical trials - carried out with the participation of a small number (less 100) healthy volunteers. The task of the testers at this stage is to verify the safety of the drug and obtain data on its metabolism., withdrawal and action of various dosages. Sometimes the phase 1 clinical trials are carried out with the participation of patients with diseases, for the treatment of which the drug is intended. At this stage, certain signs of effectiveness can be recorded., however, they are not considered statistically significant.
A new type of early clinical trial, dubbed phase 0 or microdosing, popular with developers, seeking to reduce the time and financial costs of the preclinical development stage. In such tests, no more than 10 patients, who take less 1% standard dose of the drug. Use of modern technologies, such as accelerated mass spectrometry, allows you to assess the metabolism and toxicity of the drug at the stage of the phase 0.
Phase 2
In phase 2 clinical trials usually involved 100-300 patients, suffering from the disease, for the treatment of which a new drug can theoretically be used. Wherein, in parallel with evidence of effectiveness, register additional data on the safety of the drug. Within the phase 2 researchers can simultaneously test the effectiveness of a drug against various diseases, for example, to test an anticancer drug on patients with various types of cancer. The purpose of this is to select the most appropriate population or patient populations for the phase. 3 Clinical Trials.
Phase 3
Phase 3 consists of one or more even larger tests (often around 1000-5000 patients) involving a specific population of patients with diseases, for which treatment with an experimental drug, the developers hope to get FDA approval. Phase 3 conducted to test efficacy and monitor side effects. Several tests of the same product can be carried out during this phase., applied according to one or more indications.
Process for obtaining formal approval
If clinical trials are successfully completed, the next step is to request FDA approval., which is drawn up in the form of an application for the use of a new drug (new drug application, NDA), or in the form of an application for a permit for the use of a biological product (biologics license application, BLA). Such applications can be hundreds or thousands of pages long and contain detailed information about the structure of the product., its production, results of laboratory testing and clinical trials.
As part of the execution of the Official Approval Payment Act when considering priority products (designed to address unmet medical needs) The FDA is required to respond within 6 months from the date of application. For products, considered according to the standard protocol, the time for consideration of the application should be no more than 10 Months. Term "PDUFA date" (PDUFA date) means date, to which the FDA is committed to review an application for a specific product.
When conducting an NDA or BLA assessment, especially when considering a new product, FDA may seek advice from one of its independent advisory committees. Each of these committees consists of 10-15 members of, including experts and members of the public. Committees meet in public, often in the presence of the press, where the advantages and disadvantages of the proposed product are comprehensively considered and discussed. The end result of such a meeting is a recommendation for or against the formal approval of the product..
Advisory committee recommendations, but, are not the final decision. In any event, the final decision rests with the Office..
Post-approval phase
Each approved drug receives an official instruction sheet on a standardized form., the content of which the FDA is developing in conjunction with the company, marketing the drug. The instruction contains a list of officially approved indications for use., description of the drug, possible side effects, recommended dosage, a brief description of the results of clinical trials and other information, which can be useful to the attending physicians. Doctors can prescribe drugs to treat diseases, not specified in the instructions, however, manufacturers are prohibited from selling drugs for undocumented use, and insurance does not always apply to such situations.
The story doesn't end with official approval and instructions. Companies often have additional phases 2 And 3 clinical trials to test other possible indications for drug use. Based on the results of these tests, manufacturers can apply for additional approvals in the form of NDA and BLA. If approved, new indications for use are included in the instructions..
Companies also conduct a phase 4 Clinical Trials, the purpose of which is to clarify the available data on the drug. Besides, drug manufacturers are required to inform the FDA of all identified during the phase 4 clinical trials of adverse drug effects, which are considered according to the existing rules of production and marketing.
Biotech drug development process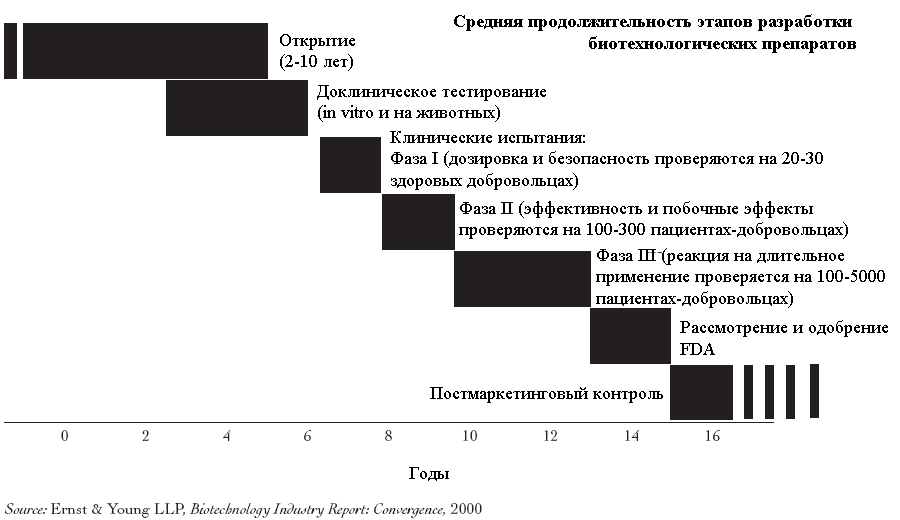
Original: cbio.ru/page/51/id/3470/
